Welcome to the website of the Sun Research Lab in the Department of Chemistry and Chemical & Biomedical Engineering at University of New Haven
Research InterestMy research seeks macromolecular approaches to solving the problems in the interface of chemistry, materials, and medicine. Several areas of synthetic polymer chemistry are being investigated, with particular focus on those most closely related to chemically-recyclable materials and biomedical applications.
|
1. Degradable and recyclable polymer materials via controlled polymerization techniques
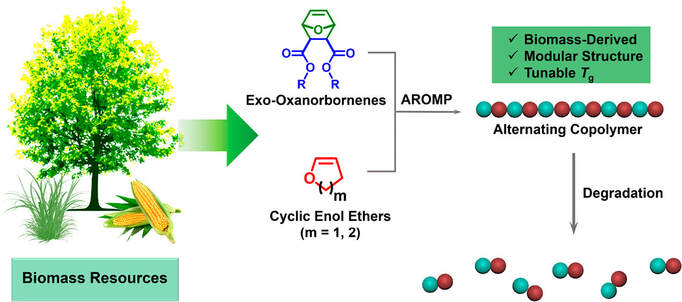
Degradable polymers made via ring-opening metathesis polymerization (ROMP) hold tremendous promise as eco-friendly materials. However, most of the ROMP monomers are derived from petroleum resources, which are typically considered less sustainable compared to biomass. Herein, we present a synthetic strategy to degradable polymers by harnessing alternating ROMP of biomass-based cyclic olefin monomers including exo-oxanorbornenes and cyclic enol ethers. A library of well-defined poly(enol ether)s with modular structures, tunable glass transition temperatures, and controlled molecular weights was achieved, demonstrating the versatility of this approach. Most importantly, the resulting copolymers exhibit high degrees of alternation, rendering their backbones fully degradable under acidic conditions.
Representative publications
1. Tarek Ibrahim, Jordan Martindale, Angelo Ritacco, Mia Rodriguez, Hao Sun*, "Polyheptenamer: A Chemically Recyclable Polyolefin Enabled by The Low Strain of Seven-Membered Cycloheptene", Journal of Polymer Science, 2024, DOI: 10.1002/pol.20240196. [Link]
2. Tarek Ibrahim, Hao Sun*, "Modular Approach to Bio-Based Poly(enol ether)s with Tunable Thermal Properties and Degradability", ACS Applied Polymer Materials, 2024, DOI: 10.1021/acsapm.3c03196. [Link]
3. Tarek Ibrahim, Angelo Ritacco, Daniel Nalley, Omar Emon, Yifei Liang, Hao Sun*, "Chemical Recycling of Polyolefins via Ring-Closing Metathesis Depolymerization", Chemical Communications, 2024, 60, 1361-1371. [Link]
4. Sun, H.*; Ibrahim, T.; Ritacco, A.; Durkee, K., "Biomass-Derived Degradable Polymers via Alternating Ring-Opening Metathesis Polymerization of Exo-Oxanorbornenes and Cyclic Enol Ethers", ACS Macro Letters, 2023, 12, 1642-1647. [Link]
5. Sun, H., Liang, Y., Thompson, M.P., Gianneschi, N.C.*, "Degradable Polymers via Olefin Metathesis Polymerization", Prog. Polym. Sci., 2021, 120, 101427. [Link]
6. Liang, Y.; Sun, H.*; Cao, W.; Thompson, M.P.; Gianneschi, N.C.*, "Degradable Polyphosphoramidate via Ring-Opening Metathesis Polymerization", ACS Macro Lett. 2020, 9, 1417-1422. [Link]
1. Tarek Ibrahim, Jordan Martindale, Angelo Ritacco, Mia Rodriguez, Hao Sun*, "Polyheptenamer: A Chemically Recyclable Polyolefin Enabled by The Low Strain of Seven-Membered Cycloheptene", Journal of Polymer Science, 2024, DOI: 10.1002/pol.20240196. [Link]
2. Tarek Ibrahim, Hao Sun*, "Modular Approach to Bio-Based Poly(enol ether)s with Tunable Thermal Properties and Degradability", ACS Applied Polymer Materials, 2024, DOI: 10.1021/acsapm.3c03196. [Link]
3. Tarek Ibrahim, Angelo Ritacco, Daniel Nalley, Omar Emon, Yifei Liang, Hao Sun*, "Chemical Recycling of Polyolefins via Ring-Closing Metathesis Depolymerization", Chemical Communications, 2024, 60, 1361-1371. [Link]
4. Sun, H.*; Ibrahim, T.; Ritacco, A.; Durkee, K., "Biomass-Derived Degradable Polymers via Alternating Ring-Opening Metathesis Polymerization of Exo-Oxanorbornenes and Cyclic Enol Ethers", ACS Macro Letters, 2023, 12, 1642-1647. [Link]
5. Sun, H., Liang, Y., Thompson, M.P., Gianneschi, N.C.*, "Degradable Polymers via Olefin Metathesis Polymerization", Prog. Polym. Sci., 2021, 120, 101427. [Link]
6. Liang, Y.; Sun, H.*; Cao, W.; Thompson, M.P.; Gianneschi, N.C.*, "Degradable Polyphosphoramidate via Ring-Opening Metathesis Polymerization", ACS Macro Lett. 2020, 9, 1417-1422. [Link]
2. Architecture-transformable polymers: a new class of stimuli-responsive polymers
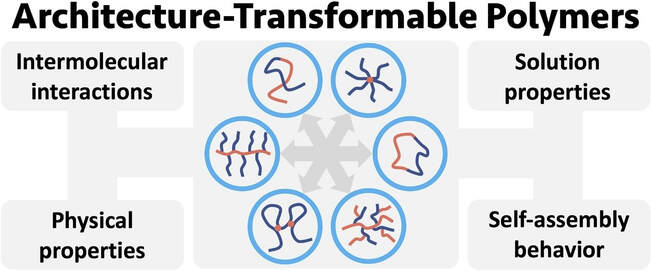
Macromolecular architecture plays a pivotal role in determining the properties of polymers. When designing polymers for specific applications, it is not only the size of a macromolecule that must be considered, but also its shape. In most cases, the topology of a polymer is a static feature that is inalterable once synthesized. Using reversible-covalent chemistry to prompt the disconnection of chemical bonds and the formation of new linkages in situ, we developed polymers that undergo dramatic topological transformations via a process we term macromolecular metamorphosis. Utilizing this technique, a linear amphiphilic block copolymer or hyperbranched polymer undergoes ‘metamorphosis’ into comb, star and hydrophobic block copolymer architectures. This approach was extended to include a macroscopic gel which transitioned from a densely and covalently crosslinked network to one with larger distances between the covalent crosslinks when heated. These architecture-transformable polymers present an entirely new approach to ‘smart’ materials.
Representative publications
1. Sun, H.; Kabb, C.P.; Sumerlin, B.S., “Thermally-labile segmented hyperbranched copolymers: using reversible-covalent chemistry to investigate the mechanism of self-condensing vinyl copolymerization”, Chem. Sci., 2014, 5, 4646-4655. [Link]
2. Sun, H.; Dobbins, D.J.; Dai, Y.; Kabb, C.P.; Wu, S.; Alfurhood, J.A.; Rinaldi, C.; Sumerlin, B.S., “Radical departure: thermally-triggered degradation of azo-containing poly(β-thioester)s”, ACS Macro lett, 2016, 5, 688-693. [Link]
3. Dai, Y.; Sun, H.; Pal, S.; Zhang, Y.; Park, S.; Kabb, C.P.; Wei, W.; Sumerlin, B.S., “Near-IR-induced dissociation of thermally-sensitive star polymers”, Chem. Sci., 2017, 8, 1815-1821. [Link]
4. Sun, H.; Kabb, C.P.; Dai, Y.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S., “Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture”, Nature Chemistry, 2017, 9, 817-823. (Highlighted in Chemistry World 2017, February 21st). [Link]
5. Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S., “Architecture-transformable polymers: reshaping the future of stimuli-responsive polymers”, Prog. Polym. Sci., 2019, 89, 61-75. (ESI highly cited paper) [Link]
1. Sun, H.; Kabb, C.P.; Sumerlin, B.S., “Thermally-labile segmented hyperbranched copolymers: using reversible-covalent chemistry to investigate the mechanism of self-condensing vinyl copolymerization”, Chem. Sci., 2014, 5, 4646-4655. [Link]
2. Sun, H.; Dobbins, D.J.; Dai, Y.; Kabb, C.P.; Wu, S.; Alfurhood, J.A.; Rinaldi, C.; Sumerlin, B.S., “Radical departure: thermally-triggered degradation of azo-containing poly(β-thioester)s”, ACS Macro lett, 2016, 5, 688-693. [Link]
3. Dai, Y.; Sun, H.; Pal, S.; Zhang, Y.; Park, S.; Kabb, C.P.; Wei, W.; Sumerlin, B.S., “Near-IR-induced dissociation of thermally-sensitive star polymers”, Chem. Sci., 2017, 8, 1815-1821. [Link]
4. Sun, H.; Kabb, C.P.; Dai, Y.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S., “Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture”, Nature Chemistry, 2017, 9, 817-823. (Highlighted in Chemistry World 2017, February 21st). [Link]
5. Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S., “Architecture-transformable polymers: reshaping the future of stimuli-responsive polymers”, Prog. Polym. Sci., 2019, 89, 61-75. (ESI highly cited paper) [Link]
3. Synthetic biomolecule-polymer conjugates
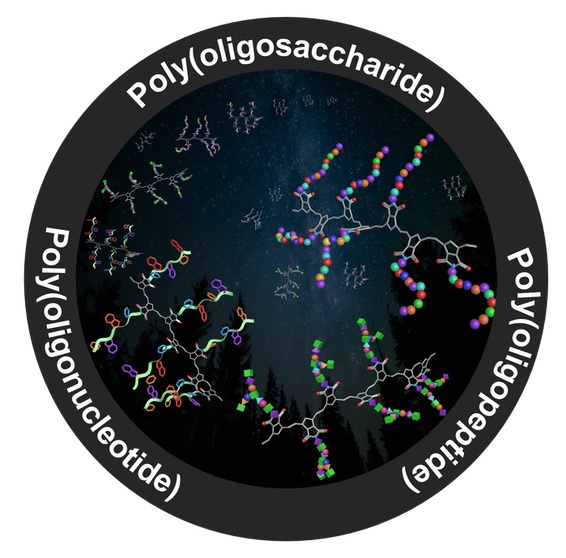
Synthetic biomolecules including oligonucleotides, oligosaccharides, and oligopeptides represent powerful therapeutics because of their biocompatibility, straightforward synthesis, predictable metabolism, and high degree of modularity in molecular design. However, these advantages are typically compromised by natural processes prevalent in cells and tissues that have evolved to degrade them. Moreover, cell internization of peptides is typically inefficient, often requiring selective cell surface interactions through the use of cell penetrating sequences. These inherent downsides of oligopeptides as drugs have tremendously hampered their translations into clinic use. To tackle these challenges, we sought to develop straightforward synthetic approaches to well-defined biomolecule-polymer conjugates via controlled/living polymerization methods. Leveraging photoinduced reversible-deactivation radical polymerization (photo-RDRP), we gained peptide brush polymers that exhibit both enhanced proteolytic resistance and improved cell uptake.
Representative publications
1. Yang, L.; Sun, H.; Liu, Y.; Hou, W.; Yang, Y.; Cai, R.; Cui, C.; Zhang, P.; Pan, X.; Li, X.; Li, L.; Sumerlin, B.S.; Tan, W., “Self-assembled aptamer-hyperbranched polymer nanocarrier for targeted and photoresponsive drug delivery”, Angew. Chem. Int. Ed., 2018, 57, 17048-17052. [Link]
2. Sun, H.; Choi, W.; Zang, N.; Battistella, C.; Thompson, M.P.; Cao, W.; Zhou, X.; Forman, C.; Gianneschi, N.C., “Bioactive Peptide Brush Polymers via Photoinduced Reversible-Deactivation Radical Polymerization”, Angew. Chem. Int. Ed., 2019, 58, 17359-17364 (Selected for Back cover). [Link]
3. Sun, H.; Yang, L; Thompson, M. P; Schara, S; Cao, W; Choi, W; Hu, Z; Zang, N; Tan, W; Gianneschi, N.C., “Recent Advances in Amphiphilic Polymer-Oligonucleotide Nanomaterials via Living/Controlled Polymerization Technologies”, Bioconjug. Chem., 2019, 30, 7, 1889-1904. [Link]
4. Choi, W.; Sun, H.; Battistella, C.; Berger, O.; Vratsanos, M.A.; Wang, M.; Gianneschi, N.C., “Biomolecular densely grafted brush polymers: oligonucleotides, oligosaccharides and oligopeptides”, Angew. Chem. Int. Ed., 2020, DOI: 10.1002/anie.202005379. [Link]
5. Zhu, J.; Sun, H.; Callmann, C.E.; Thompson, M.P.; Battistella, C.; Proetto, M.T.; Carlini, A.S.; Gianneschi, N.C., “Paclitaxel-terminated peptide brush polymers”, Chem. Commun., 2020, DOI: 10.1039/c9cc10023g. (Selected for Back cover) [Link]
6. Sun, H., Cao, W., Zang, N., Clemons, T. D., Scheutz, G. M., Hu, Z., Thompson, M. P., Liang, Y., Vratsanos, M., Zhou, X., Choi, W., Sumerlin, B. S., Stupp, S. I, Gianneschi, N. C. “Proapoptotic Peptide Brush Polymer Nanoparticles via Photoinitiated Polymerization-Induced Self-Assembly.” Angew. Chemie. Int. Ed. 2020, DOI: 10.1002/anie.202006385. [Link]
1. Yang, L.; Sun, H.; Liu, Y.; Hou, W.; Yang, Y.; Cai, R.; Cui, C.; Zhang, P.; Pan, X.; Li, X.; Li, L.; Sumerlin, B.S.; Tan, W., “Self-assembled aptamer-hyperbranched polymer nanocarrier for targeted and photoresponsive drug delivery”, Angew. Chem. Int. Ed., 2018, 57, 17048-17052. [Link]
2. Sun, H.; Choi, W.; Zang, N.; Battistella, C.; Thompson, M.P.; Cao, W.; Zhou, X.; Forman, C.; Gianneschi, N.C., “Bioactive Peptide Brush Polymers via Photoinduced Reversible-Deactivation Radical Polymerization”, Angew. Chem. Int. Ed., 2019, 58, 17359-17364 (Selected for Back cover). [Link]
3. Sun, H.; Yang, L; Thompson, M. P; Schara, S; Cao, W; Choi, W; Hu, Z; Zang, N; Tan, W; Gianneschi, N.C., “Recent Advances in Amphiphilic Polymer-Oligonucleotide Nanomaterials via Living/Controlled Polymerization Technologies”, Bioconjug. Chem., 2019, 30, 7, 1889-1904. [Link]
4. Choi, W.; Sun, H.; Battistella, C.; Berger, O.; Vratsanos, M.A.; Wang, M.; Gianneschi, N.C., “Biomolecular densely grafted brush polymers: oligonucleotides, oligosaccharides and oligopeptides”, Angew. Chem. Int. Ed., 2020, DOI: 10.1002/anie.202005379. [Link]
5. Zhu, J.; Sun, H.; Callmann, C.E.; Thompson, M.P.; Battistella, C.; Proetto, M.T.; Carlini, A.S.; Gianneschi, N.C., “Paclitaxel-terminated peptide brush polymers”, Chem. Commun., 2020, DOI: 10.1039/c9cc10023g. (Selected for Back cover) [Link]
6. Sun, H., Cao, W., Zang, N., Clemons, T. D., Scheutz, G. M., Hu, Z., Thompson, M. P., Liang, Y., Vratsanos, M., Zhou, X., Choi, W., Sumerlin, B. S., Stupp, S. I, Gianneschi, N. C. “Proapoptotic Peptide Brush Polymer Nanoparticles via Photoinitiated Polymerization-Induced Self-Assembly.” Angew. Chemie. Int. Ed. 2020, DOI: 10.1002/anie.202006385. [Link]